“There is now too much pressure on the review of innovative medical devices in the medical device field, for example, some enterprises told us, ‘You must approve us, otherwise, we will lose our series A funding’ or ‘If you don’t approve our products for marketing, all our investors will fail and we will be finished’,” said Sun Lei from the Center for Medical Device Evaluation, NMPA (“CMDE”) recently at the Summit Forum on Medical Device Innovation Development and Scientific Supervision (“Summit”).
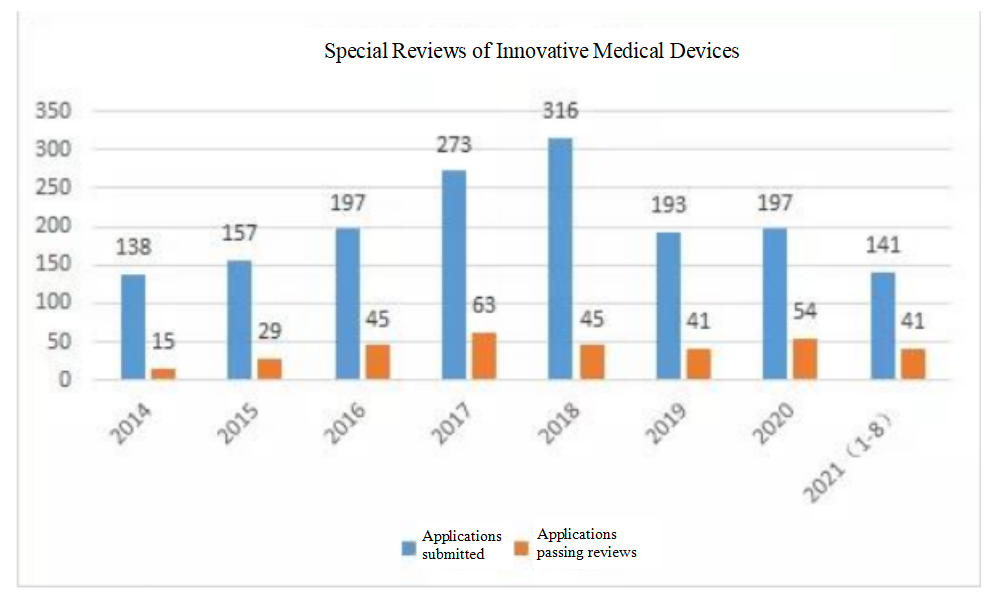
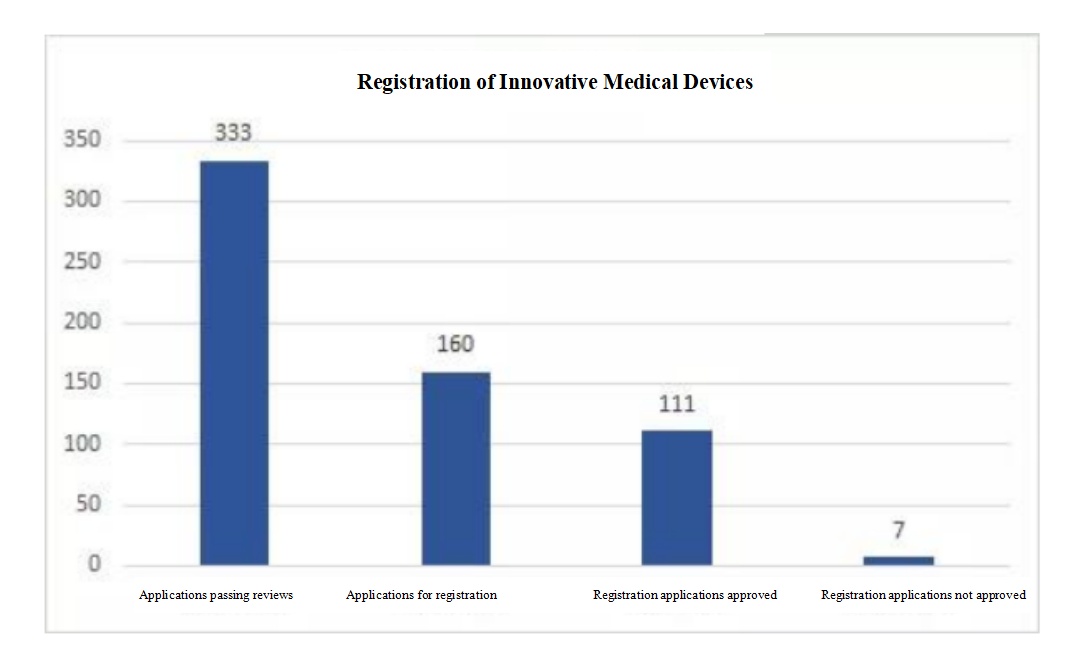
At the Summit, Director Sun Lei presented a summary report on the data of the current special review of innovative medical devices: since the implementation of the special review procedures for innovative medical devices in 2014, 1,612 applications for special reviews of innovative products were received by August 23, 2021, 333 passed reviews, accounting for 20.7%; among the 333, 160 (177 registration units) have entered the approval procedures, and 111 (119 registration units) have been approved for registration; among the 119 products approved for registration and marketing, 44% are active products, 38% are passive products, and 18% are IVD products, including 114 domestic ones and five imported ones.
Director Sun Lei also mentioned that the CMDE’s priority support for innovative medical devices is reflected in each review and approval process; the Regulation on the Supervision and Administration of Medical Devices (“Regulation”) and the Measures for the Supervision and Administration of Medical Devices have been comprehensively revised and reformed in recent years, and many aspects of the review and approval system have been optimized, for example, medical device innovation is included in the development priorities; innovative medical devices are given priority review and approval; the promotion and use of innovative medical devices are supported; the high-quality development of the medical device industry is promoted.
He also pointed out that the current policy and system optimization is also reflected in terms of product registration, clinical trial, registrant and filing party systems, etc., and the reform of these systems will help encourage enterprises to conduct R&D and innovation.
With priority policies and support in place, what are the future goals and directions of innovation?
The Outline of the 14th Five-Year Plan emphasizes seven key areas for future sci-tech breakthroughs, including artificial intelligence, quantum information, integrated circuits, brain science and brain-inspired technology, genetic and life technologies, clinical medicine and health, deep holes and deep sea and polar exploration.
Sun Lei said that many of these key areas are closely related to healthcare, and China will accelerate the domestic substitution of high-end medical equipment to reduce costs, promote the constant development of national brand enterprises, and also accelerate remedying shortcomings in Chinese high-end medical equipment and making breakthroughs in key and core technologies, and break technology and equipment bottlenecks, to achieve independent control of high-end medical equipment.
With policies and goals, how to advance innovative development?
General Secretary Xi Jinping pointed out the need to focus on world-level cutting-edge technologies, the main economic fields, the country’s critical needs, and the people's health.
Director Sun said, in this regard, the government should optimize the resource allocation mechanism and sci-tech achievement transformation mechanism and focus on the combination of government-industry-academia-research synergy mechanisms, for example, establish high-level sci-tech innovation platforms and state-level key laboratories, increase financial investment, and improve policy guidance,
while from the perspective of enterprises, he said, “Enterprises are always the main body of medical device innovation and the main force to promote innovation. Enterprises should aim the right direction, increase investment, equip resources, and prepare sufficient funds, which is the best path to achieve innovation because innovation is inseparable from funding.”
Positive and negative effects of capital
When talking about the status of innovation investment and financing in recent years, he said, “Medical device innovation has been exceptionally active in recent years and attracting a large influx of social capital, and a common phenomenon at present is that investors seek to invest in enterprises instead of the other way around. Capital is certainly important to innovation, but we need to pay attention to its negative effects. The massive influx of social capital will promote increased innovation activity of enterprises, on the one hand, but it will also lead to prolonged survival of innovation projects without value, uneven quality of innovation projects, and sluggish demand for high-quality development, on the other hand. We need to view this double-edged sword rationally.”
He also said, “There is now too much pressure on the review of innovative medical devices in the medical device field, for example, some enterprises told us, ‘You must approve us, otherwise, we will lose our series A funding’ or ‘If you don’t approve our products for marketing, all our investors will fail and we will be finished’.” The involvement of capital makes reviews of innovative medical devices exceptionally difficult, however, innovation must be premised on product safety and effectiveness.
Regarding the continued encouragement of enterprise innovation and high-quality industry development, Director Sun stressed that the CMDE’s reform initiatives will continue to focus on optimizing the allocation of review resources for innovative medical devices and adjusting the approval strategy.
He said that first of all, the review focus should be shifted, problems that hinder the development should be solved, and early involvement and whole-process guidance should be provided for projects of products with breakthroughs in core technologies and key components. “To really solve the problems that hinder the development, we need true innovation instead of superficial innovation. A device in a domestically produced housing but with foreign core components is not called innovation,” said Sun Lei. The CMDE will fully implement the reform requirements for the clinical evaluation of medical devices according to the new edition of Regulation, continue to optimize the review mechanism and process, promote the implementation of medical device master file registration and other systems that encourage medical device innovation, establish medical device innovation cooperation platforms in different fields, and give play to state strength to support the innovative development of medical devices.
Source: Innomd.org
Editor: Wu Hong
At the Summit, Director Sun Lei presented a summary report on the data of the current special review of innovative medical devices: since the implementation of the special review procedures for innovative medical devices in 2014, 1,612 applications for special reviews of innovative products were received by August 23, 2021, 333 passed reviews, accounting for 20.7%; among the 333, 160 (177 registration units) have entered the approval procedures, and 111 (119 registration units) have been approved for registration; among the 119 products approved for registration and marketing, 44% are active products, 38% are passive products, and 18% are IVD products, including 114 domestic ones and five imported ones.

Special Reviews of Innovative Medical Devices

Registration of Innovative Medical Devices
Director Sun Lei also mentioned that the CMDE’s priority support for innovative medical devices is reflected in each review and approval process; the Regulation on the Supervision and Administration of Medical Devices (“Regulation”) and the Measures for the Supervision and Administration of Medical Devices have been comprehensively revised and reformed in recent years, and many aspects of the review and approval system have been optimized, for example, medical device innovation is included in the development priorities; innovative medical devices are given priority review and approval; the promotion and use of innovative medical devices are supported; the high-quality development of the medical device industry is promoted.
He also pointed out that the current policy and system optimization is also reflected in terms of product registration, clinical trial, registrant and filing party systems, etc., and the reform of these systems will help encourage enterprises to conduct R&D and innovation.
With priority policies and support in place, what are the future goals and directions of innovation?
The Outline of the 14th Five-Year Plan emphasizes seven key areas for future sci-tech breakthroughs, including artificial intelligence, quantum information, integrated circuits, brain science and brain-inspired technology, genetic and life technologies, clinical medicine and health, deep holes and deep sea and polar exploration.
Sun Lei said that many of these key areas are closely related to healthcare, and China will accelerate the domestic substitution of high-end medical equipment to reduce costs, promote the constant development of national brand enterprises, and also accelerate remedying shortcomings in Chinese high-end medical equipment and making breakthroughs in key and core technologies, and break technology and equipment bottlenecks, to achieve independent control of high-end medical equipment.
With policies and goals, how to advance innovative development?
General Secretary Xi Jinping pointed out the need to focus on world-level cutting-edge technologies, the main economic fields, the country’s critical needs, and the people's health.
Director Sun said, in this regard, the government should optimize the resource allocation mechanism and sci-tech achievement transformation mechanism and focus on the combination of government-industry-academia-research synergy mechanisms, for example, establish high-level sci-tech innovation platforms and state-level key laboratories, increase financial investment, and improve policy guidance,
while from the perspective of enterprises, he said, “Enterprises are always the main body of medical device innovation and the main force to promote innovation. Enterprises should aim the right direction, increase investment, equip resources, and prepare sufficient funds, which is the best path to achieve innovation because innovation is inseparable from funding.”
Positive and negative effects of capital
When talking about the status of innovation investment and financing in recent years, he said, “Medical device innovation has been exceptionally active in recent years and attracting a large influx of social capital, and a common phenomenon at present is that investors seek to invest in enterprises instead of the other way around. Capital is certainly important to innovation, but we need to pay attention to its negative effects. The massive influx of social capital will promote increased innovation activity of enterprises, on the one hand, but it will also lead to prolonged survival of innovation projects without value, uneven quality of innovation projects, and sluggish demand for high-quality development, on the other hand. We need to view this double-edged sword rationally.”
He also said, “There is now too much pressure on the review of innovative medical devices in the medical device field, for example, some enterprises told us, ‘You must approve us, otherwise, we will lose our series A funding’ or ‘If you don’t approve our products for marketing, all our investors will fail and we will be finished’.” The involvement of capital makes reviews of innovative medical devices exceptionally difficult, however, innovation must be premised on product safety and effectiveness.
Regarding the continued encouragement of enterprise innovation and high-quality industry development, Director Sun stressed that the CMDE’s reform initiatives will continue to focus on optimizing the allocation of review resources for innovative medical devices and adjusting the approval strategy.
He said that first of all, the review focus should be shifted, problems that hinder the development should be solved, and early involvement and whole-process guidance should be provided for projects of products with breakthroughs in core technologies and key components. “To really solve the problems that hinder the development, we need true innovation instead of superficial innovation. A device in a domestically produced housing but with foreign core components is not called innovation,” said Sun Lei. The CMDE will fully implement the reform requirements for the clinical evaluation of medical devices according to the new edition of Regulation, continue to optimize the review mechanism and process, promote the implementation of medical device master file registration and other systems that encourage medical device innovation, establish medical device innovation cooperation platforms in different fields, and give play to state strength to support the innovative development of medical devices.
Source: Innomd.org
Editor: Wu Hong
